UNDERSTANDING CRYSTALLIZATION OF HONEY
The Myths That Keep Good Honey away from Mainstream Market
What comes to mind when you see grainy or semi-solid or crystallized honey? You might suspect it to be fake or adulterated honey, but the truth is quite opposite, Crystallization of honey is an indication of purity and rawness of honey.
After extraction from the comb, natural honey crystallizes sooner or later. This process may take days, months, or even years to occur. The speed of crystallization depends on various factors, which we’ll discuss in detail here.
What is Crystallization of Honey?

Crystallization is the process in which liquid honey naturally turns into granules or becomes semi-solid. The white crystals may resemble table sugar, leading some to mistakenly believe that sugar has been added to the honey. However, this is not the case. Crystallization is a natural property of pure honey and is a sign of its authenticity. It does not compromise the honey’s quality or alter its taste.
Why It happens ?
Natural honey is a supersaturated solution of monosaccharides, primarily dextrose (glucose) and levulose (fructose). Dextrose, due to its lower solubility in water, begins to settle and crystallize over time. Once crystallization starts, each crystal acts as a nucleus, promoting further crystallization. This process continues naturally, resulting in liquid honey turning to a granulated or semi-solid state.
Texture of Honey After Crystallization
Complete and Partial Crystallization

Honey crystallization can sometimes occur partially, where only the bottom portion of the jar crystallizes first. This process may continue until the entire jar solidifies or may remain partially crystallized, depending on various factors.
Most of the time, only partial crystallization occurs, creating an interesting contrast between the liquid and solid sections in the jar. However, there are cases where the entire jar crystallizes, as seen with mustard honey, which tends to crystallize completely.
Smooth or Grainy Texture
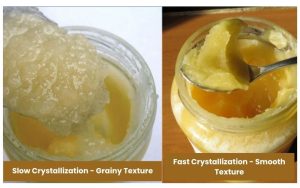
The texture of crystallized honey can vary from smooth and creamy to coarse and grainy.
This variation is influenced by the rate of crystallization. When crystallization happens rapidly, it forms tiny, uniform crystals, resulting in a smooth, buttery texture. Conversely, slow crystallization produces larger crystals, leading to a coarse, grainy feel.
Factors such as temperature, the type of nectar source, and storage conditions play a significant role in determining the rate of crystallization and the resulting texture of the crystallized honey.
Honey preserves itself through the process of crystallization
Crystallization acts as a natural preservation process for honey, helping it retain its nutrients over thousands of years. In fact, archaeologists have discovered honey in Egyptian pyramids that, despite being thousands of years old, was still edible due to its crystallized state.
Factors Affecting speed of Crystallization of Honey
Nectar source.

The speed of crystallization depends on the source of the flower from which honey bees collect nectar. For example, if honey bees collect nectar majorly from Mustard flowers, the honey is likely to crystallize faster, whereas Acacia and Jamun flower honey take several months or longer to turn into crystallize state.
This is due to the varying composition of monosaccharides in honey. If dextrose (glucose) is more abundant in the nectar, the honey will crystallize faster. On the other hand, if levulose (fructose) is present in higher amounts, the crystallization process will take longer.
Temperature

Temperature plays a crucial role in the speed at which honey crystallizes. Lower temperatures often encourage crystallization, while higher temperatures inhibit it. This is due to basic physics—dissolution increases when a liquid is warm, making it less likely for the sugars in honey to settle and crystallize.
That is why we often see the instruction on Honey label “Do Not Refrigerate Honey”
Pollen and other particles in Honey
Small amounts of pollen, air bubbles, and other impurities in honey act as ‘nucleation seeds,’ influencing its crystallization. These impurities create inhomogeneity within the honey, lower the surface energy barrier, and initiate crystal formation (nucleation), which then promotes the growth of additional crystals and accelerates crystallization.
This phenomenon is also utilized to produce intentional or planned crystallized honey called Cream honey or Whipped. where honey is deliberately crystallized by mixing already crystallized honey as seed or nuclei for smooth texture which is easy to spread on bread.
Immobility time
Immobility time is another factor that affects the crystallization of honey. When honey is in frequent use, it crystallizes more slowly because the movement disrupts the formation of stable crystals. Crystals need time to settle and grow, so honey that is left undisturbed will crystallize faster.
Crystallization Note on Honey Label
Did you know? Most honey labels around the world inform consumers about the natural process of crystallization. Take a moment to check the label on the honey jar sitting on your shelf. You’ll likely find a statement similar to: “Crystallization of honey is a natural phenomenon, especially in colder conditions. Do not refrigerate honey. If it crystallizes, place the jar in warm water or sunlight to restore its liquid state.” It’s surprising that even well-educated consumers often overlook this information and mistakenly view crystallized honey as fake or adulterated.
Why The Myth is so strong Despite Clear information on label?
Why does some honey crystallize and others don’t ?
Many of us notice that some types of honey crystallize while others remain liquid, even when refrigerated, especially popular branded honey.
As discussed, the varying nectar sources can be one reason for this difference. However, in the case of popular branded honey that does not crystallize, the cause is often harsh processing. This includes high heating and ultrafiltration, which remove pollen and other natural particles that would otherwise act as nuclei for crystallization. A recent and concerning trend is the use of advanced adulterants, such as rice syrup or high-fructose syrups, which do not crystallize.
What’s the best way to de-crystallize honey?
To turn crystallized honey back into its liquid state, place the sealed jar in a bowl of warm water. The gentle heat will gradually dissolve the sugar crystals. Ideally around 40°C – 50°C warm water is enough. It’s important to be patient during this process it may take a long time to transfer heat to middle portion of honey, stirring can also be done if there is provision. Alternatively honey can be kept in sunlight for whole day.
Conclusion
While many consumers mistakenly associate crystallization with impurity, it is actually a sign of pure, unprocessed honey. Commercial practices that prevent crystallization, such as high heating, ultrafiltration, and the use of adulterants, compromise the natural properties and nutritional value of honey. Embracing crystallized honey ensures that you enjoy honey in its most wholesome, nutrient-rich form.
 Watch this video to learn more and enjoy your honey with confidence!
Watch this video to learn more and enjoy your honey with confidence!
Frequently Asked Question
- Dextrose to Fructose Ratio: Crystallizes much sooner in that honey containing high dextrose or glucose. This one containing much levulose or fructose as Acacia and Jamun takes long to crystallize.
- Temperature: Crystallization can be expedited through cooling temperatures. If it has stored at the fridge or any such cold location then it crystallizes the soonest.
- Nectar Source: Flowers of different nectar compositions yield honey containing different proportions of glucose and fructose. Mustard honey is crystallized more rapidly, while litchi honey crystallizes slower.
- Immobilization: Honey not stirred or otherwise used tends to crystallize.
- People ate honey immediately after extracting it. For centuries, people have traditionally stored honey in its liquid form, thanks to preservation techniques that help maintain its smooth, pourable texture.
- Commercial brands ensure that their honey stays liquid with the help of processing techniques. This gives the customers an impression that liquid honey is better.
- Many companies are taking advantage of this myth to sell synthetic or processed honey that remains liquid for a longer time and has no health benefits associated with raw honey.

